Quality Management
Basic Approach
In accordance with our corporate philosophy and Sustainability Policy, we define our Basic Policy for Quality Assurance as follows.
- 1We contribute to society by providing high-quality products, goods and services with excellent reliability and safety to the market.
- 2We fully understand and predict user demands and provide products, goods and services that users can use with satisfaction.
- 3In each quality assurance step, we establish an internal system that can assuredly evaluate high-quality products, goods and services with excellent reliability and safety.
- 4We establish technologies that can produce products, goods and services with the target quality in terms of reliability and safety.
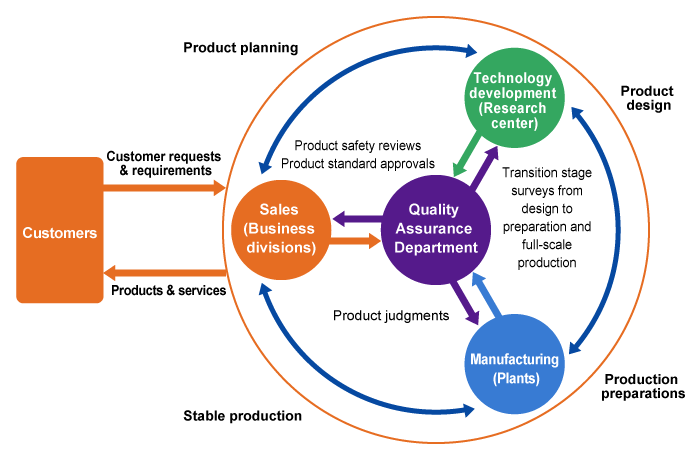
Structure and System
In order to consistently manufacture products that reflect the needs and wants of customers and ensure product quality, Zeon is advancing integrated quality assurance activities Group-wide, across manufacturing, sales, and engineering functions, by strengthening cooperation between plants, departments and research units (R&D Center).
Key Initiatives
| Initiatives (topics) |
Targets | Results | Status |
|---|---|---|---|
| Timely responses to complaints, opinions from customers, etc. | Complete responses within a single year | A case exceeding a single year occurred; however, it was completed in April 2025. Internal regulations were also revised around the same time to prevent recurrence. | ✓ |
| Enhance customer satisfaction | Analysis on customer satisfaction and initiatives for improvements | In order to efficiently analyze customer satisfaction surveys, a new system was introduced, and the identification of issues was initiated company-wide and within each division. | ✓ |
Framework supporting quality assurance
In order to ensure a consistent supply of high-quality products to our customers, we have put in place various quality assurance systems in accordance with internal regulations on quality assurance and conforming to the ISO 9001:2015 international standard for quality management systems.
In 2010, we obtained Group-wide consolidated ISO 9001 certification and have maintained the certification since then. Zeon Group companies have also obtained global standard certifications related to quality.
In addition, in order to prevent quality issues from occurring, we offer commentaries on actual case studies for issues that could potentially arise as part of annual e-learning compliance checks to confirm levels of understanding for all employees. We are working to ensure that every employee has an accurate understanding of quality assurance and performs their duties.
Going forward, in order to establish a quality assurance system that adapts to changes in the external environment, we plan to switch our ISO 9001 certification from Group-wide certification to worksite-specific certification.
Status of Zeon Group certifications for international quality standards
| Site | ISO 9001:2015*1 | ISO 13485:2016*2 | IATF 16949:2016*3 | FSSC 22000v5*4 |
|---|---|---|---|---|
| Japan | ||||
| Zeon Corporation | ✓ | ✓*6 | ||
| Zeon Kasei Co., Ltd. | ✓*5 | |||
| Zeon Polymix Inc. | ✓ | |||
| Zeon Opto Bio Lab Co., Ltd. | ✓ | ✓ | ||
| Tokyo Zairyo Co., Ltd. | ✓ | |||
| Tohpe Corporation | ✓ | |||
| Zeon Medical Inc. | ✓ | |||
| Zeon North Co., Ltd. | ✓ | |||
| Zeon Chemicals Yonezawa Co., Ltd. | ✓ | ✓ | ||
| Outside Japan | ||||
| Zeon Chemicals L.P. | ✓ | |||
| Zeon Chemicals (Thailand) Co., Ltd. | ✓ | |||
| Zeon Advanced Polymix Co., Ltd. | ✓ | ✓ | ||
| Zeon Chemicals Singapore Pte. Ltd. | ✓ | |||
| Zeon Europe GmbH | ✓ | |||
| Zeon Chemicals Asia Co., Ltd. | ✓ | |||
| Zeon Kasei (Changshu) Co., Ltd. | ✓ | |||
| Zeon Kasei Mexico S.A. de C.V. | ✓ | |||
- *1ISO 9001 is the international standard to increase customer satisfaction based on efforts to guarantee product and service quality through continuous improvement of quality management systems.
- *2ISO 13485 is the global standard for quality management systems in the field of medical devices for the purpose of continued manufacture and provision of safe and useful medical devices. The standard has added requirements specific to medical devices while omitting some of the ISO 9001 requirements.
- *3IATF 16949 is a standard issued by the International Automotive Task Force (IATF) for the automobile industry sector, and which is intended as a supplement to the requirements of ISO 9001 for automobile manufacturers.
- *4FSSC 22000 (Food Safety System Certification 22000) is a standard developed by the Foundation of Food Safety Certification to certify management systems for the production of safe food.
- *5The logistical materials division has acquired certification limited to certain shipping containers.
- *6Mizushima Plant has acquired FSSC 22000 certification for the manufacture of food-grade synthetic aroma chemicals.
Framework to achieve product safety
- 1. Product safety reviews
- We strive to ensure product safety in every possible aspect by conducting PSR* using our own checklists that consider product safety at every stage of the product lifecycle, from initial product development through planning, design, manufacturing, sales, use, and disposal.
- *PSR: Product safety review
- 2. Chemical substance regulatory compliance
- The regulatory environment for chemical substances management is undergoing major change globally, with laws and regulations on chemical substances being enacted and amended not just in the United States and Europe but also in Japan and Southeast Asia. As a result, the number of regulations to comply with is rising sharply.
At Zeon, we are creating a database of the substances in our raw materials and products, even those present in minute quantities, and building a chemical substances management system capable of continuously tracking the most up-to-date regulatory information, safety information, and other relevant information.
Audits
- PL audits
- PL audits consist of individually performed audits at business departments and plants using checklists, while on-site audits target product liability (PL) and product safety and are performed by an audit team led by a compliance officer. PL audits are conducted for product liability activities for products of Zeon business divisions spanning all stages of development, manufacture, use, final consumption, and disposal.
- Quality audits
- Quality audits are conducted when serious issues concerning quality arise, performed by an auditing team led by the head of the Quality Assurance Department that focuses on checking the progress of implementing quality improvement activities. They are conducted at Zeon’s business divisions, plants, laboratories, and Group companies.
- Internal quality audits
-
Internal quality audits are conducted for the purposes of evaluating whether Zeon’s quality assurance management system is operating effectively and efficiently based on ISO 9001: 2015 and promoting ongoing improvements. The audits are based on ISO 9001 and are structured so that the requirements of ISO 9001 are met as long as Zeon’s internal regulations are being complied with. The audits also focus on customer satisfaction and overall optimization.
The audits are conducted for the whole Zeon Group; as far as possible, audits are conducted by an auditing team comprised of auditors from other divisions. We identify issues through internal quality audits and connect these activities to improvements in our systems and processes.
Quality data management
At Zeon, we define all customer comments or expressions of dissatisfaction with our products or services as “complaints,” and utilize a complaints response system to report, review, approve, and manage delivery time.
We have also implemented a specification management system to ensure compliance with the customer specifications, by aligning customer specifications and product test criteria.
Inspection data is further checked against product test criteria in the product testing system, with a decision of pass/fail assigned, and results structured to be automatically forwarded to the ERP system.
Process changes and process abnormalities are also reliably managed through the deployment of a system that reports, reviews, and sends out effectiveness evaluations, emergency measures, and corrective actions, to ensure the implementation of assured change management and deviation management. We are currently constructing an even more reliable and efficient system for quality assurance by means of linking these systems.
Risk reduction for new products
Zeon conducts a comprehensive review (PSTR)*1 from quality assurance perspectives when transitioning from the production preparations stage to actual production.
This review confirms items related to product quality to ensure that new products satisfy the quality requirements of our customers, and to allow us to fulfill supply obligations. Other items subject to review include data on the chemical substances making up the product; 3D-QFD (Quality Function Deployment), for data-based clarification of cause-and-effect relationships of information related to manufacturing; FMEA*2, which attempts to prevent potential post-production abnormalities; and testing methodologies and testing facilities to measure quality characteristics and characteristics of critical processes.
- *1PSTR: Product Stage-gate Transfer Review
- *2FMEA: Failure Mode and Effects Analysis
Communicating safety information
For more information about safety information for chemical products handled by Zeon, see Home > Products/ Business > SDS・chemSHERPA.
