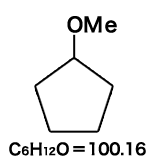
Cyclopentyl methyl ether (CPME)
Cyclopentyl methyl ether (CPME) is a totally new hydrophobic ether solvent, which was established out of ZEON's unique synthetic technology and C5 raw materials.
Unlike other common ether solvents, CPME has unique excellent properties and is widely applicable as a replacement for Tetrahydrofuran (THF), Methyl Tert-Butyl Ether (MTBE), Dioxane and other existing ether solvents.
- TSCA
- Approved
- EC Number
- 445-090-6
- Japan
- 3-4548
Benefits
- HIGH HYDROPHOBICITY
Easy separation and recovery from water, reducing emissions and wastewater
Wide applicability as a reaction, extraction and crystallization solvent, giving simple and one-pot syntheses - WIDE LIQUIDITY RANGE
Wide applications from lower to higher temperature, accelerating reaction rate - LOW HEAT OF VAPORIZATION
Saving energy for distillation and recovery - RESIST PEROXIDE FORMATION
Low exothermic decomposition energy of solvent containing it's peroxides - NARROW EXPLOSION AREA
- RELATIVELY STABLE TO ACIDS OR BASES
- EASY DRYING
Applications
- REACTIONS
Grignard Reaction, Suzuki Coupling, Buchwald Amination, Metal Reductions (NaBH4, LiAlH4, i-Bu2AlH), Reactions with n-BuLi, Reactions with Lewis Acid, Friedel Crafts Reactions - EXTRACTIONS
- CRYSTALLIZATIONS
- POLYMERIZATIONS
- COATINGS
Physical Properties
| CPME | MeTHF | THF | Diethyl ether |
Dioxane | MTBE | |
|---|---|---|---|---|---|---|
| Relative density | 0.86 | 0.85 *d | 0.89 *a | 0.71 *b | 1.03 *a | 0.70 *a |
| Vapor specific gravity (air=1) |
3.45 | 2.97 *d | 2.5 *a | 2.6 *a | 3.0 *a | 3.0 *a |
| Boiling point [℃] | 106 | 80.2 *d | 66 *a | 35 *a | 101 *a | 55 *a |
| Melting point [℃] | <-140 | -136 *d | -108.5 *a | -116 *a | 12 *a | -109 *a |
| Viscosity(20℃)[cP] | 0.57 | 0.46 *d(25ºC) | 0.55 *b | 0.24 *b | 1.31 *b | |
| Surface tension(20℃)[mN/m] | 25.17 | 26.4 *b | 17.3 *b | 36.9 *b | ||
| Heat of vaporization (boiling point)[Kcal/kg] |
69.2 | 87.1 *d | 98.1 *b | 86.1 *b | 98.6 *b | |
| Specific heat(20℃)[Kcal/kg・K] | 0.435 | 0.469 *b | 0.584 *c | 0.41 *b | ||
| Dielectric constant(25℃) | 4.76 | 6.97 *d | 7.58 *b | 4.20 *b | 2.24 *b | |
| Azeotropic temperature with water [℃] | 83 | 71 *d | 64 *c | 34 *b | 88 *b | |
| Azeotropic composition ( Solvent / Water, wt% ) | 83.7 / 16.3 | 89.4 / 10.6 *d | 94.0 / 6.0 *c | 98.7 / 1.3 *b |
81.6 / 18.4 *b | |
| Solubility in water(23℃)[g/100g] | 1.1 | 14 *d (20ºC) | ∞ *a | 6.9 *a (20ºC) | ∞ *a | 4.2 *a |
| Solubility of water in solvent(23℃)[g/100g] | 0.3 | 4 *d (20ºC) | ∞ *b | 1.2 *b | ∞ *b | |
| Flash point [℃] | -1 | -11 *d | -14.5 *a | -45 *a | 12 *a | -28 *a |
| Auto Ignition temperature [℃] | 180 | 270 *e | 321 *a | 160 ~180 *a |
180 *a | 375 *a |
| Explosion range [Vol%] Lower limit | 1.1 | 1.5 *e | 2 *a | 1.7 *a | 2 *a | 1.6 *a |
| Explosion range [Vol%] Upper limit | 9.9 | 8.9 *e | 11.8 *a | 48 *a | 22 *a | 15.1 *a |
- *aInternational Chemical Safety Cards(ICSC)
- *b溶剤ハンドブック、講談社(1989)
- *c溶剤ポケットブック、オーム社(2001)
- *dOrg. Process Res. Dev.,2007, 11(1), pp156-159
- *ePenn A Kem, Metyhtetrahydrofran, MSDS Date:10/1/2010
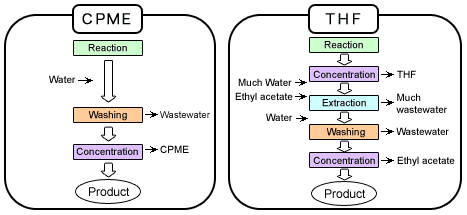
Organic Processes with CPME vs. THF
CPME simplifies processes, reduces wastewater and emissions.
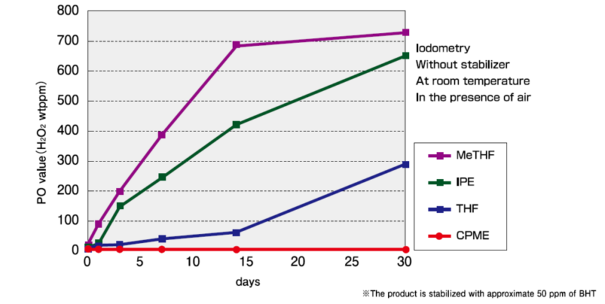
Peroxide Formation of Ether Solvents
Product Specifications
- purity(GC)
- 99.90% min
- Water
- 100ppm max
- Color(Hazen No)
- 10 max
- Peroxide
- 50wtppm max
Packings
170kg Drum, 16kg Can
Cautions
- The information contained herein is believed to be reliable, but no representations, guarantees or warranties of any kind are made as to its accuracy, stability for particular applications or results to be obtained.
- Please read the Safety Data Sheet (SDS) carefully prior to handling.
- This product was developed for the applications herein. In case of other applications, please handle under your confirmation of safety for the applications, or please talk to Zeon Corporation beforehand.
References
1.Cyclopentyl Methyl Ether: An Elective Ecofriendly Ethereal Solvent in Classical and Modern Organic Chemistry
Prof. Dr. Ugo Azzena ; Dr. Massimo Carraro ; Dr. Luisa Pisano ; Serena Monticelli ;
Dr. Roberta Bartolotta ; Prof. Dr. Vittorio Pace. Cyclopentyl Methyl Ether: An Elective Ecofriendly Ethereal Solvent in Classical and Modern Organic Chemistry. CHEMSUSCHEM. Vol 12, January 2019, P.40-70
2.Grignard Reactions in Cyclopentyl Methyl Ether
Dr. Shoji Kobayashi ; Keisuke Shibukawa ; Yuta Miyaguchi ; Prof. Dr. Araki Masuyama.
Grignard Reactions in Cyclopentyl Methyl Ether. Asian Journal of Organic Chemistry.
Vol 5, May 2016, P.636-645